Low solar activity and colder climates pose an increased pandemic influenza risk during this grand solar minimum. Highly pathogenic avian H7N9, H5N1, and other influenza-A strains have already killed more than 2,000 humans worldwide since 2003. Worryingly since 2013, a newly emerging highly pathogenic avian H7N9 influenza strain has killed over 1,600 Chinese people in a growing epidemic each year.
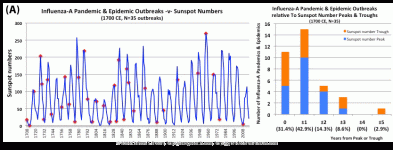
Pandemic influenza outbreaks bias sunspot number peaks and troughs
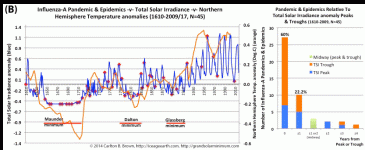
This grand solar minimum portends pandemic influenza outbreaks
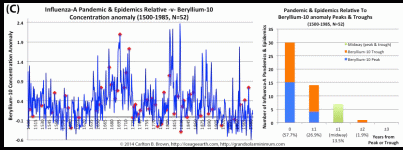
Solar activity peaks and troughs portend pandemic influenza outbreaks
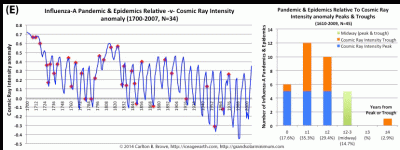
Cosmic ray intensity peaks and troughs portend pandemic influenza outbreaks
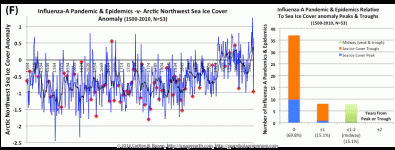
Extremes of Arctic sea ice extent portend pandemic influenza outbreaks
These increased pandemic influenza risks make it a priority to pre-immunize the world’s population against high-risk, potentially pandemic H7N9 and H5N1 influenza-A viruses. These two influenza-A strains are knocking loudly at our species’ door; they have killed between 25 percent and 50 percent of infected humans (the percentage may be even higher).[1],[2],[3],[4],[5] A highly lethal pandemic flu outbreak could kill between one and three percent of the world’s population. This was the mortality rate during the 1918–1919 Spanish flu pandemic.[6]
For all diseases that can be prevented by vaccination, it is both absolutely imperative and the norm to immunize people before a disease emerges.[7],[8],[9] However, under the existing paradigm of pandemic influenza immunization and supply, governments and WHO wait until a pandemic emerges before calling for the manufacture of a pandemic flu vaccine to immunize the population. Under this paradigm, sufficient quantities of vaccine cannot be manufactured quickly enough[10],[11] to protect the world’s population before the peak of a pandemic’s mortality is reached.[12]
Since the 2009 swine flu pandemic, vaccine technology advances offering immunological protection against ongoing viral mutation have made it possible to immunize people ahead of the outbreak of a pandemic (i.e., pre-pandemic immunization).[13] An upgraded flu vaccine technology would permit a broader immunity against emerging pandemic flu strain mutants, and enable us to better protect the population before the outbreak of a pandemic.[14],[15],[16],[17],[18],[19],[20],[21],[22] Why haven’t we upgraded the flu vaccine technology and implemented pre-pandemic immunization? You can read Chapter 14 of “Revolution: Ice Age Re-Entry” to find out more.
Developing nations produce a significant share of the world’s food. Look at the top five grain exporters in the following citation and you will see the importance of developing nations to grain supply and global food security.[23] Developing nations will be hit hardest by vaccine supply inequities, because their people will not be immunized in time under the current plan for supplying pandemic flu vaccine.[24],[25],[26] People living in developed nations will be hard pressed as well, unless their governments have stockpiled sufficient quantities of a pre-pandemic flu vaccine (unlikely).
There is clearly a need for change, what I term a pandemic flu vaccine revolution. With a pandemic flu vaccine revolution our governments could ensure we are protected, and our fragile global economy and the global food supply are insured against the increased pandemic influenza risk. Pre-pandemic immunization makes good sense before a climate switch and the increased pandemic flu risks it portends.
[1] S. Yamayoshi et al., “Enhanced Replication of Highly Pathogenic Influenza A(H7N9) Virus in Humans.” Emerging Infectious Diseases Journal. 2018;24(4):746-750. https://dx.doi.org/10.3201/eid2404.171509.
[2] Qi Tang et al., “China is closely monitoring an increase in infection with avian influenza A (H7N9) virus.” BioScience Trends. 2017; 11(1):122-124. DOI: 10.5582/bst.2017.01041.
[3] Artois J et al., “Changing Geographic Patterns and Risk Factors for Avian Influenza A(H7N9) Infections in Humans, China.” Emerging Infectious Diseases Journal 2018;24(1):87-94. https://dx.doi.org/10.3201/eid2401.171393
[4] Centers for Disease Control and Prevention. “Highly Pathogenic Asian Avian Influenza A (H5N1) in People.” https://www.cdc.gov/flu/avianflu/h5n1-people.htm
[5] L.O. Durand et al., “Timing of Influenza A(H5N1) in Poultry and Humans and Seasonal Influenza Activity Worldwide, 2004–2013.” Emerging Infectious Diseases Journal. 2015;21(2):202-208. https://dx.doi.org/10.3201/eid2102.140877.
[6] Centers for Disease Control and Prevention. First Global Estimates of 2009 H1N1 Pandemic Mortality Released by CDC-Led Collaboration. 1918 Spanish flu mortality rate estimates. https://www.cdc.gov/flu/spotlights/pandemic-global-estimates.htm.
[7] Roy M Anderson and Robert M May, “Vaccination and Herd Immunity to Infectious Diseases.” Nature, Volume 318, 28 November 1985. DOI: 10.1038/318323a0.
[8] T.J. John and R. Samuel, “Herd immunity and herd effect: new insights and definitions.” European Journal of Epidemiology (2000) 16: 601. https://doi.org/10.1023/A:1007626510002.
[9] T.H. Kim et al., “Vaccine herd effect.” Scandinavian Journal of Infectious Diseases. 2011;43(9):683-689. doi:10.3109/00365548.2011.582247.
[10] R.H. Borse et al., “Effects of Vaccine Program against Pandemic Influenza A(H1N1) Virus, United States, 2009–2010.” Emerging Infectious Diseases Journal. 2013;19(3):439-448. https://dx.doi.org/10.3201/eid1903.120394. https://wwwnc.cdc.gov/eid/article/19/3/12-0394_article#r3.
[11] Xiao-Feng Liang et al., “Safety of Influenza A (H1N1) Vaccine in Postmarketing Surveillance in China.” New England Journal of Medicine 364;7 nejm.org February 17, 2011.
[12] Harvey V. Fineberg, “Pandemic Preparedness and Response — Lessons from the H1N1 Influenza of 2009.” April 3, 2014. New England Journal of Medicine2014; 370:1335-1342. DOI: 10.1056/NEJMra1208802.
[13] Rino Rappuoli and Philip R. Dormitzer, “Influenza: Options to Improve Pandemic Preparation.” Science 22 Jun 2012: Volume 336, Issue 6088, 1531-1533. DOI: 10.1126/science.1221466.
[14] Ali H. Ellebedy et al., “H5N1 immunization broadens immunity to influenza.” Proceedings of the National Academy of Sciences Sep 2014, 111 (36) 13133-13138; DOI: 10.1073/pnas.1414070111.
[15] Paul Gillard et al., “Long-term booster schedules with AS03A-adjuvanted heterologous H5N1 vaccines induces rapid and broad immune responses in Asian adults.” BMC Infectious Diseases201414:142. https://doi.org/10.1186/1471-2334-14-142.
[16] Isabel Leroux-Roels et al., “Broad Clade 2 Cross-Reactive Immunity Induced by an Adjuvanted Clade 1 rH5N1 Pandemic Influenza Vaccine.” PLOS. Published: February 27, 2008. https://doi.org/10.1371/journal.pone.0001665.
[17] Grazia Galli et al., “Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine.” Proceedings of the National Academy of Sciences May 2009, 106 (19) 7962-7967; DOI:10.1073/pnas.0903181106.
[18] Lopez P et al., “Combined Administration of MF59-Adjuvanted A/H5N1 Prepandemic and Seasonal Influenza Vaccines: Long-Term Antibody Persistence and Robust Booster Responses 1 Year after a One-Dose Priming Schedule.” Clinical and Vaccine Immunology : CVI. 2013;20(5):753-758. doi:10.1128/CVI.00626-12.
[19] Banzhoff A et al., “MF59®‐adjuvanted vaccines for seasonal and pandemic influenza prophylaxis. Influenza and Other Respiratory Viruses.” 2008;2(6):243-249. doi:10.1111/j.1750-2659.2008.00059.x.
[20] P Gillard et al., “An assessment of prime‐boost vaccination schedules with AS03A‐adjuvanted prepandemic H5N1 vaccines: a randomized study in European adults.” Influenza and Other Respiratory Viruses. 2013;7(1):55-65. doi:10.1111/j.1750-2659.2012.00349.x.
[21] Anuradha Madan et al., “Immunogenicity and Safety of an AS03-Adjuvanted H7N9 Pandemic Influenza Vaccine in a Randomized Trial in Healthy Adults.” The Journal of Infectious Diseases, Volume 214, Issue 11, 1 December 2016, 1717–1727, https://doi.org/10.1093/infdis/jiw414.
[22] Stadlbauer D et al., 2017, “Vaccination with a recombinant H7 hemagglutinin-based influenza virus vaccine induces broadly reactive antibodies in humans.” mSphere 2:e00502-17. https://doi.org/10.1128/mSphere.00502-17.
[23] Christopher Bren d’Amour et al., “Teleconnected food supply shocks.” Environment Research Letter 11 (2016) 035007 doi:10.1088/1748-9326/11/3/035007. (see table 1, page 2 for the list of countries for each main crop).
[24] Martin Enserink, “The Challenge of Getting Swine Flu Vaccine to Poor Nations.” Question and Answer session; discussing vaccine supply versus serving national interests first. Nov. 3, 2009 , 3:22 PM . Accessed 01/04/2018. http://www.sciencemag.org/news/2009/11/challenge-getting-swine-flu-vaccine-poor-nations.
[25] Mark Turner, 2015, “Vaccine procurement during an influenza pandemic and the role of Advance Purchase Agreements: Lessons from 2009-H1N1.” Global Public Health, 11:3, 322-335, DOI: 10.1080/17441692.2015.1043743.
[26] D.P. Fidler, 2010, “Negotiating Equitable Access to Influenza Vaccines: Global Health Diplomacy and the Controversies Surrounding Avian Influenza H5N1 and Pandemic Influenza H1N1.” PLoS Med 7(5): e1000247. https://doi.org/10.1371/journal.pmed.1000247.