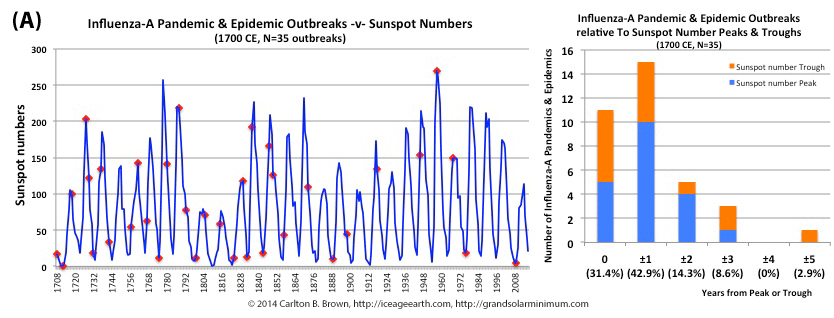
Pandemic influenza outbreaks bias sunspot number peaks and troughs
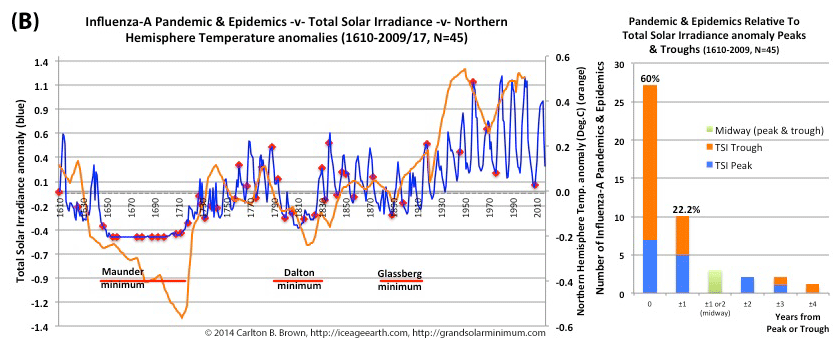
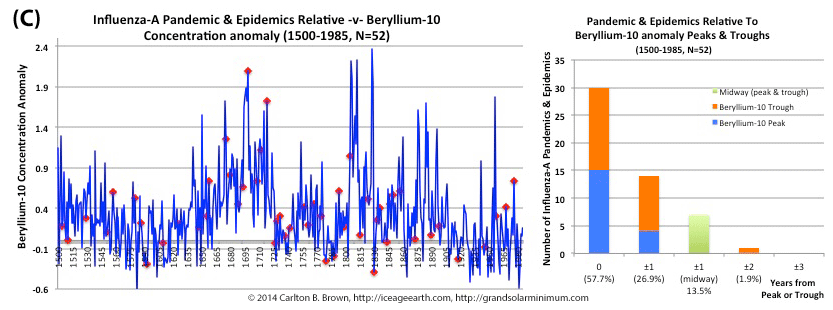
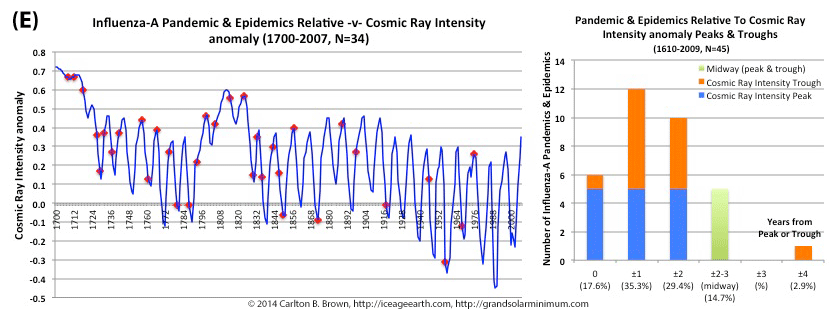
Figure A. Historical pandemic and epidemic influenza-A outbreak data was extracted from six scientific publications reviewing the history of influenza, providing a general consensus on pandemic flu outbreaks (and major regional epidemics) back to 1500. These were plotted against observed sunspot numbers. See the citation for all data.[i] Seventy-four percent of influenza pandemics and epidemics (26/35 events) since 1700 CE occurred at or within one year of the peak or trough in sunspot numbers, increasing to 89 percent (31/35) within two years. The average sunspot number for pandemics occurring at sunspot number troughs was 12 (18 for pandemics occurring within one year of a sunspot number trough). The 2018 sunspot number was 22.
Based on sunspot numbers, we are approaching a high-risk period for pandemic flu. This increased risk is given more gravity, considering half of all pandemics since 1600 CE occurred in the trough of grand solar minima. The sun has already entered a grand solar minimum.
The influenza-A viruses we really have to worry about are highly pathogenic avian influenza-A H7N9 and H5N1. Since 1997 other animal influenza-A viruses have also killed humans, and these continue to pose risks.[ii],[iii],[iv],[v]
H7N9 is killing between 25 and 40 percent of humans infected.[vi],[vii] Animal-to-human infections emerged in China in 2013, and grew year by year to a total of 1,600 animal-to-human infections reported by 2017.[viii],[ix],[x] Specific viral mutations that facilitate human infection have since emerged,[xi] meaning human-to-human transmission is next. The situation is similar with the H5N1 virus, which has killed more than 50 percent of humans infected.[xii],[xiii],[xiv],[xv]
Pandemics have historically spread rapidly throughout the world, and up to half the human population is typically infected.[xvi],[xvii],[xviii],[xix] Pandemic flu viruses that kill a high percentage of their victims do so because they cause a high incidence of severe pneumonia and multi-organ failure. This requires intensive hospital care, with the availability of hospital intensive care a potential bottleneck.
The 1918–1919 pandemic flu virus caused acute swelling of and bleeding from the lungs, and people who were infected typically suffocated within one to two days. The second wave of the pandemic was responsible for the most deaths, due to an unusually severe hemorrhagic pneumonia. H5N1 victims today experience similar pathologies to those of the 1918–1919 pandemic, with acute respiratory distress syndrome occurring in 50 to 75 percent of infections.[xx],[xxi]
Likewise, since 2013 more than 90 percent of humans dying from H7N9 infection suffered from pneumonia, respiratory failure, or acute respiratory distress syndrome. Most of the infected people who were hospitalized were admitted to an intensive care unit. With ongoing viral mutations of H7N9 known to improve human viral transmission,[xxii] this is a very worrying virus indeed.
Click on this page and download a free copy of my book “Revolution: Ice Age Re-Entry,” and read more about this topic in Chapter 14.
[i] Data: (1) Yearly mean total sunspot number (1700 – 2017). Sunspot data from the World Data Center SILSO, Royal Observatory of Belgium, Brussels. http://sidc.be/silso/datafiles#total. Downloaded 05/05/2018. (2) Influenza pandemic and epidemic publications: (a) B. Lina, 2008, History of Influenza Pandemics. In: Raoult D., Drancourt M. (eds) Paleomicrobiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75855-6_12. (b) E. Tognotti, 2009, Influenza pandemics: a historical retrospect. Journal of Infection in Developing Countries, 3:331-334. doi: https://doi.org/10.3855/jidc.239. (c) C. Potter, 2001, A history of influenza. Journal of Applied Microbiology, 91: 572-579. doi:10.1046/j.1365-2672.2001.01492.x. (d) J.K. Taubenberger and D.M. Morens, 1918 Influenza: the Mother of All Pandemics. Emerging Infectious Diseases. 2006;12(1):15-22. doi:10.3201/eid1201.050979. (e) Edwin D. Kilbourne, Influenza. Chapter 1; History of Influenza. Springer Science & Business Media, 6/12/2012 – Medical. ISBN 978-1-4684-5239-6. (f) Svenn-Erik Mamelund, Influenza, Historical. December 2008. International Encyclopedia of Public Health, First Edition (2008), vol. 3, pp. 597-609. DOI: 10.1016/B978-012373960-5.00372-5..
[ii] J.K. Taubenberger and D.M. Morens, “Pandemic influenza – including a risk assessment of H5N1.” Revue scientifique et technique (International Office of Epizootics). 2009;28(1):187-202.
[iii] M. Gilbert et al., “Climate change and avian influenza.” Revue scientifique et technique (International Office of Epizootics). 2008;27(2):459-466.
[iv] Mark A. Miller et al., “The Signature Features of Influenza Pandemics —Implications for Policy.” New England Journal of Medicine2009; 360:2595-2598. DOI: 10.1056/NEJMp0903906.
[v] Paul Gillard et al., “Long-term booster schedules with AS03Aadjuvanted heterologous H5N1 vaccines induces rapid and broad immune responses in Asian adults.” BMC Infectious Diseases201414:142. https://doi.org/10.1186/1471-2334-14-142.
[vi] Hai-Nv Gao et al., “Clinical Findings in 111 Cases of Influenza A (H7N9) Virus Infection.” New England Journal of Medicine2013; 368:2277-2285. DOI: 10.1056/NEJMoa1305584.
[vii] Qi Tang et al., “China is closely monitoring an increase in infection with avian influenza A (H7N9) virus.” BioScience Trends. 2017; 11(1):122-124. DOI: 10.5582/bst.2017.01041.
[viii] Yamayoshi S et al., “Enhanced Replication of Highly Pathogenic Influenza A(H7N9) Virus in Humans.” Emerging Infectious Diseases Journal 2018;24(4):746-750. https://dx.doi.org/10.3201/eid2404.171509.
[ix] European Centre for Disease Prevention and Control. “Human infection with avian influenza A(H7N9) virus–fifth update.” 27 February 2017. Stockholm: ECDC; 2017.
[x] Artois J et al., “Changing Geographic Patterns and Risk Factors for Avian Influenza A(H7N9) Infections in Humans, China.” Emerging Infectious Diseases Journal 2018;24(1):87-94. https://dx.doi.org/10.3201/eid2401.171393.
[xi] N. Xiang et al., “Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic — China.” September 2015–August 2016. MMWR Morb Mortal Wkly Rep 2016;65:1390–1394. DOI: http://dx.doi.org/10.15585/mmwr.mm6549a2.
[xii] U.S. Department of Health and Human Services. Pandemic Influenza Plan 2017 Update. https://www.cdc.gov/flu/pandemic-resources/pdf/pan-flu-report-2017v2.pdf.
[xiii] Centers for Disease Control and Prevention. “Highly Pathogenic Asian Avian Influenza A (H5N1) in People.” https://www.cdc.gov/flu/avianflu/h5n1-people.htm.
[xiv] Kumnuan Ungchusak et al., “Probable Person-to-Person Transmission of Avian Influenza A (H5N1). 2005.” New England Journal of Medicine2005; 352:333-340. DOI: 10.1056/NEJMoa044021.
[xv] L.O. Durand et al., “Timing of Influenza A(H5N1) in Poultry and Humans and Seasonal Influenza Activity Worldwide, 2004–2013.” Emerging Infectious Diseases Journal 2015;21(2):202-208. https://dx.doi.org/10.3201/eid2102.140877.
[xvi] B. Lina, 2008, “History of Influenza Pandemics.” In: Raoult D., Drancourt M. (eds) Paleomicrobiology. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-540-75855-6_12.
[xvii] E. Tognotti, “Influenza pandemics: a historical retrospect.” The Journal of Infection in Developing Countries 2009, 3: 331-334. DOI: https://doi.org/10.3855/jidc.239.
[xviii] C. Potter, 2001, “A history of influenza.” Journal of Applied Microbiology, 91: 572-579. doi:10.1046/j.1365-2672.2001.01492.x.
[xix] J.K. Taubenberger and D.M. Morens, “1918 Influenza: the Mother of All Pandemics.” Emerging Infectious Diseases. 2006;12(1):15-22. doi:10.3201/eid1201.050979.
[xx] Eugenia Tognotti, “Emerging Problems in Infectious Diseases Influenza pandemics: a historical retrospect.” The Journal of Infection in Developing Countries2009; 3(5):331-334.
[xxi] Yu-Chia Hsieh et al., “Influenza Pandemics: Past, Present and Future.” J. Formos Med Assoc. 2006 Jan;105(1):1-6. DOI:10.1016/S0929-6646(09)60102-9.
[xxii] Xiang N et al., “Assessing Change in Avian Influenza A(H7N9) Virus Infections During the Fourth Epidemic — China.” September 2015–August 2016. MMWR Morb Mortal Wkly Rep 2016;65:1390–1394. DOI: http://dx.doi.org/10.15585/mmwr.mm6549a2.

Recent Comments